E and Z Notation For Alkenes
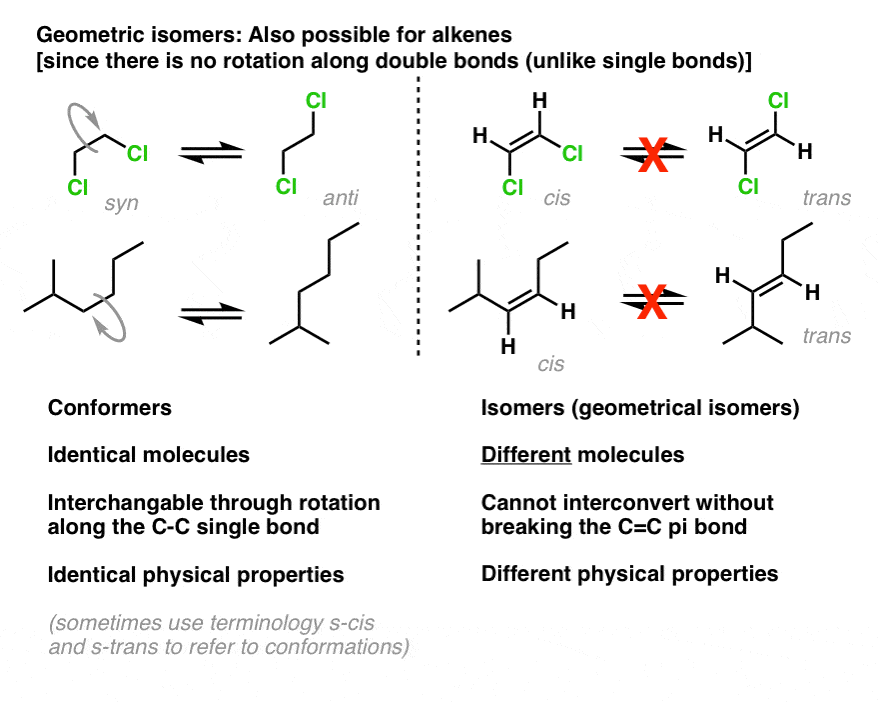
- Unlike C–C single bonds, C–C double bonds can’t undergo rotation without breaking the pi bond
- One consequence of this is geometric isomerism –the existence of alkene stereoisomers that differ solely in how their substituents are arranged in space about the double bond
- In simple cases where there are two identical substituents on each carbon of the alkene, we can usecis– andtrans– to designate the isomers where those substituents are on thesameandopposite sides of the double bond, respectively.
- For geometric isomers that lack two identical substituents, we rank the two substituents on each end of the double bond according to the Cahn-Ingold-Prelog (CIP) rules.
- TheZ isomer (“zusammen“, same) is the geometric isomer where the #1 ranked substituents are on the same side of the double bond. Mnemonic: “zee zame zide“
- E isomer (“entgegen“) is the geometric isomer where the #1 ranked substituents are on the opposite side of the double bond,
Table of Contents
- When do we use cis– andtrans– Notation In Rings?
- cis– and trans– Isomerism In Alkenes
- Watch out for ambiguous names when geometrical isomerism is possible!
- cis– and trans– isomerism in cyclic alkenes
- When “cis“- and “trans‘” fails: E and Z Notation
- E and Z Notation For Alkenes
- Breaking Ties: The Method of Dots
- Conclusion: E and Z Notation For Alkenes
- Notes
- Quiz Yourself!
This post was co-authored with Matt Pierce ofOrganic Chemistry Solutions. Ask Matt about scheduling an online tutoring sessionhere.
Quick Review: cis– Andtrans- Isomerism (“Geometrical Isomerism”) In Rings
Earlier on our MOC series on cycloalkanes, we saw that akey feature of small rings is that they can’t be turned “inside out” without breaking bonds.(See post: Cycloalkanes – Dashes and Wedges)
One of the most important consequence of this is that it can lead to the existence of stereoisomers –molecules which share the same molecular formula and the same connectivity but have a different arrangement of atoms in space.
These two versions of1,2 dichlorocyclopentane (below) are an example.They have the same connectivity – both are 1,2-dichlorocyclopentane – but have differentarrangements of their atoms in space. The chlorines are on the same side of the ring in the left-hand isomer (both “wedges”, coming out of the page) and on the opposite sides (one wedged, one dashed) on the right-hand isomer.

These two molecules cannot be interconverted through rotation of the C-C bond without rupturing the ring (use a model kit and try, if you like). They are thereforeisomers.
Molecules which have the same connectivity but different arrangement in space are known asstereoisomers.
Specifically, the relationship between the two molecules above is that ofdiastereomers: stereoisomers which are not mirror images of each other. (See post: Types of Isomers)
These two molecules have different physical properties – different boiling points, melting points, reactivities, spectral characteristics and so on.
[Just to note, the other subclass of stereoisomer is “enantiomers”. We apply this to two stereoisomers which are (non-superimposable) mirror images of each other.Also:keep in mind that the terms “diastereomer” and “enantiomer” denote comparative relationships, like the terms “brother” or “cousin”. ]
1. When Do We Usecis-Andtrans-Notation In Rings?
We use the terms cis- andtrans– to denote therelativeconfiguration of two groups to each other in situations where there is restricted rotation.
[Side note: the “restricted rotation” is how cis- andtrans-subtly differs from syn andanti,which we usein cases where there is free rotation, such as the orientation of methyl groups in “eclipsed” and “staggered” butane. Bottom line:syn andantiformscan generally be interconverted through bond rotation: cis and transforms cannot.]
In nomenclature, “cis” is used to distinguish the isomer where two identicalgroups (e.g. the two chlorines in 1,2-dichlorocyclopentane) are pointing in the same direction from the plane of the ring, and trans to distinguish the isomer where they point in opposite directions. [You might also hear organic chemists say, “the chlorines arecis to each other” or “the hydrogens aretrans to one another”.]
A common name for these so-called “cis-trans” isomers is “geometric isomers”. Those scolds at IUPAC actually discourage the term “geometric isomers”, and for once, I agree: the term is somewhat redundant and can cause confusion. In the rest of this post I’ll just use the term “cis-trans” isomers.
In order forcis- trans-isomerism to exist in rings, we need two conditions:
- two (and only two) carbons eachbearing non-identical substituents above and below the ring
- the two carbons have at least one of those substituents in common
In 1,2-dichlorocyclopentane we saw that C-1 and C-2 each had non-identical substituents (H and Cl) above and below the ring, and they each had at least one substituent in common (in fact they have two substituents in common: H and Cl ).
Here’s another example: cis-andtrans– 1-ethyl-2-methylcyclobutane. Note that they each have two carbons which each bear non-identical substituents above and below the ring (H and CH3; H and CH2CH3). They also have at least one substituent in common (H). So we can refer tocis-1-ethyl-2-methylcyclohexane as the isomer where the two hydrogens are pointing in the same direction, andtrans where they point in opposite directions.

If you’ve covered chirality, you might also note an interesting fact: there are two ways to draw each of thecis-andtrans– isomers, and they can’t be superimposed on each other. These areenantiomers, by the way. (See post: Enantiomers, Diastereomers or the Same)
Socis-andtrans- doesn’t specify which enantiomer (it can be applied to either). It’s just describing therelative configuration of the two groups (Hin this case). If we want to specify a particular enantiomer, we need to use the Cahn-Ingold-Prelog (CIP) system of assigning R and S configurations,which provides us with the “absolute” configuration. In that case, cis– andtrans-is redundant. (See post: Cahn-Ingold-Prelog System)
Becausecis– andtrans– is relative, it doesn’t workif the two carbons don’t share a common substituent. In that case you also have to use(R)/(S).
We’re taking too long to go through rings here, so let’s just illustrate 2 examples where “cis” and trans” doesn’t work in rings and leave it there.

2. cis– and trans- Isomerism (Geometric Isomerism) In Alkenes
cis-trans isomerismis also possible for alkenes. As in small rings, rotation about pi bonds is also constrained: due to the “side-on” overlap of pi bonds, one can’t rotate a pi bond without breaking it. This stands in contrast to conventional sigma bonds (single bonds) in acyclic molecules, where free rotation is possible: witness 1,2-dichloroethane (below left).
Hence we can have molecules such as cis-1,2-dichloroethene [boiling point 60°C] andtrans-1,2-dichloroethene [boiling point: 48°C] which can be separated from each other due to their differing physical properties.
We can also use thecis–transnomenclature to distinguish isomers such as 2-methyl-3-hexene (above right). In thecis isomer, the two hydrogens are on the same side of the pi bond, and in thetrans isomer, the two hydrogens are on the opposite side of the bond. [Note: this risks a “tsk-tsk” with accompanying finger-wag from IUPAC , but it nevertheless gets the right structure: see the Note 1 below for a digression as to why]
As with rings, the minimum requirement forcis-transisomerism in alkenes is thateach carbon is bonded to two different groups,and thatthe two carbons have at least one substituent in common.
As with rings, cis-transisomerism isn’t possible if one of the carbons of the double bond is attached to two identical groups, as with 1,1-dibromo-1-propene, below. Try it for yourself if you’re not convinced.

3. Watch Out For Ambiguous Names Where Cis/Trans Isomerism Is Possible
A quick digression: oneconsequence of our newfound appreciation of geometrical isomerism is that manysimple-sounding molecule names are actually ambiguous.
For instance, the descriptor “3-hexene” does not unambiguouslydescribe a specific molecule. [The same is true for 2-butene: try it! ]. To nail down the specific molecule, we need to specifycis– ortrans– 3-hexene.

Note that 1-hexene is still OK, since the 1-position of 1-hexene is attached to two identical groups (hydrogens) and thus nocis–trans isomers are possible.
4. Cis– Trans- Isomerism For Cyclic Alkenes
cis- andtrans can also beapplied to alkenes in rings. For example,on paper it’s possible to drawcis– andtrans– cyclohexene, since the pi bond fulfills the requirements forcis- trans-isomerism.In reality,trans-cyclohexene is impossibly strained. Try kissing yourself on the tailbone. That will give you some idea of the strain involved in trying to accommodate a trans– double bond ina six membered ring .[Note 2]
For this reason, for ring sizes 7 and below, it’s safe to ignore writing “cis” : the configuration is assumed.

At ring sizes of 8 and above, wedo need to put acis– ortrans-in the name, because thetrans– isomer becomes feasible. (Imaginetrying to kiss yourself on the tailbone if you had the neck of a giraffe: suddenly not impossible!)

5. A Solution For When “Cis” and “Trans” Fails: The E/Z System
We saw that cis and trans fails in rings when the two carbons lacked a common substituent. It also fails for alkenes under these circ*mstances.
Case in point: try to applycis andtrans to the alkene below:

See the problem?
In the absence of two identical groups, we have no reference point!
On the left, the chlorine iscis to Br andtransto F. But does that really justify calling the isomer “cis” ? How do we decide?
What we need is some way to determinepriorities in these situations.
[note: some textbooks may still refer to this alkene as exhibiting “cis-trans isomerism” even though we must use E and Z]
6. The E and ZNotation For Alkenes
Thankfully, we can apply the ranking system developed by Cahn, Ingold, and Prelog for chiralcenters (as touched on in this earlier post on (R)/(S) nomenclature) for this purpose.
The protocol is as follows:
- Each carbon in the pi bond is attached to two substituents. For each carbon, these twosubstituents areranked (1 or 2) according to the atomic numbers of the atom directly attached to the carbon. (e.g. Cl > F )
- If both substituents ranked 1 are on the same side of the pi bond, the bond is given the descriptorZ(short for GermanZusammen, which means “together”).
- If both substituents ranked 1 are on theopposite sideof the pi bond, the bond is given the descriptorE(short forGerman Entgegen, which means “opposite”).
SoZ resembles“cis” andE resembles“trans” .(Note: they arenot necessarily the same and do not always correlate: see Note 2 for an example of a cis alkene which isE .TheE/Z system iscomprehensivefor all alkenes capable of geometric isomerism, including thecis/trans alkene examples above. We oftenusecis/trans for convenience, butE/Z is the “official”, IUPAC approved way to name alkene stereoisomers].
One easy way to rememberZ is to say “Zee Zame Zide” in a German accent.My way of doing it waspretending that the Z stands for“zis”.Whatever works for you.
Here’s a practical example:

As with chiral centers, ranking according to atomic number can result in ties if we restrict ourselves merely to the atoms directly attached to the pi bonds.
7. Breaking Ties: The Method of Dots
For instance, the alkene below presents us with a dilemma: one of the carbons of the alkene is attached to two carbon atoms. So how do we determine priorities in this case. How do we break ties?

In the case of ties, we must apply themethod of dots. Dots are handy placeholders which is why I like to use this method.
- Place a dot on each of the two atoms you are comparing.
- List the 3 atomseach atom isattached to, in order of atomic number.
- Compare the lists, much like youwould compare a set of three playing cards. Just as a hand of (8, 8, 7) would beat (8, 7, 7), so would (C, C, H) beat (C, H, H).
- If the lists are identical, move the dots outward to the highest priority atom on the list.
- At the first point of difference,assign (E orZ).
- If there is no difference… then the groups are identical, andE / Zdoes not apply.
Here’s a practical example of the “method of dots”.
Here’s a more complex example with multiple alkenes. In this case each pi bond is designated by a number with its own separateE orZ configuration.

OK, this was long. But hopefully useful.
Watch out for a future post in which we go into more detail on the “method of dots”.
8. Conclusion: E and Z Notation For Alkenes
cis-trans- is OK for describing simple alkene stereoisomers, but only works in certain cases. Furthermore, itonly gives relative configurations.TheE/Zsystem is comprehensive and describes theabsoluteconfiguration of the molecule.
See below for an example of anEalkene which is “cis” and aZ alkene which is“trans”.
Just a reminder: this postwas co-authored by Matt Pierce ofOrganic Chemistry Solutions. Ask Matt about scheduling an online tutoring sessionhere.
Notes
Related Articles
- Introduction to Assigning (R) and (S): The Cahn-Ingold-Prelog Rules
- Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) – The Method of Dots
- Alkene Stability
- Types of Isomers: Constitutional Isomers, Stereoisomers, Enantiomers, and Diastereomers
- Enantiomers vs Diastereomers vs The Same? Two Methods For Solving Problems
- Geometric Isomers In Small Rings: Cis And Trans Cycloalkanes
- Stereoselective and Stereospecific Reactions
- Alkene Addition Reactions: “Regioselectivity” and “Stereoselectivity” (Syn/Anti)
Note 1: It’s possible to have an alkene we’d describe as ‘cis’beE and vice versa.
E/Zis the preferred, more comprehensive nomenclature since it describesabsolute configuration, whereascis- trans-merely describesrelativeconfiguration.

Note 2:trans-cyclopropene,trans-cyclobutene,andtrans-cyclopentene have never been synthesized or observed.trans-cyclohexene is a laboratory curiosity, stable at a few degrees above absolute zero.trans-cycloheptene has an extremely short half-life at room temperature.trans-cyclooctene is a stable molecule [it also exhibits axialchirality, which is interesting! ].
Quiz Yourself!
 Click to Flip
Click to Flip

 Click to Flip
Click to Flip

 Click to Flip
Click to Flip

 Click to Flip
Click to Flip

